Abstract
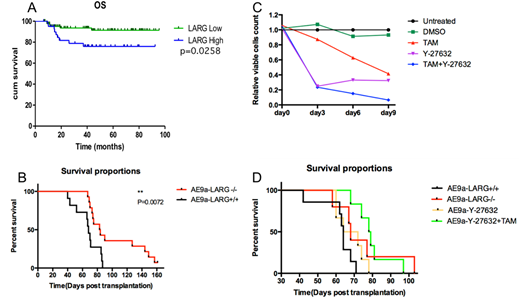
As a transcription factor AML1-ETO modulate gene expression of its targets. Scanning our ChIP-seq data we found AML1-ETO bind to an AML1 binding motif in the promotor region of ARHGEF12. Exploring acute myeloid leukemia (AML) microarray databases we confirmed that the expression of ARHGEF12 was up-regulated consistently in AML1-ETO-positive AML patient samples. To confirmed this finding, we quantitated expression of ARHGEF12 in pediatric AML samples from Shanghai children's medical center. Not out of expectation, all AML1-ETO-positive cases have higher ARHGEF12. Moreover, the ARHGEF12 expression was an independent poor prognostic factor for overall survival comparing between patients with higher and lower expressed ARHGEF12 (P=0.0258) (Figure A).
To understand the role of ARHGEF12 in leukemogenic function of AML1-ETO fusion gene, we knocked down ARHGEF12 expression in kasumi-1 cells by shRNA. Growth of kasumi-1 cells in vitro was significantly inhibited with cell cycle exit and more apoptosis. Further, we transformed fetal liver cells of Arhgef12 conditional knockout mice to leukemia model by retrovirus conducted AML1-ETO9a(AE9a) expression. Induced Arhgef12 deletion delayed full blown of leukemia in vivo. The overall survival prolonged 15.5 days (median 83.5 vs. 68 days, P=0.0072). (Figure B) ARHGEF12 is a well-known guanine nucleotide exchange factor for RhoA GTPase. Arhgef12 deletion decreased activated GTP-RhoA and MYPT1 phosphorylation, which is one of ROCK kinase substrates. Spleen cells from leukemic mice were cultured in vitro, the ROCK inhibitor Y-27632 significantly decreased the cell proliferation, which mimic the tamoxifen-induced Arhgef12 knockout phenotype. Y-27632 combined with tamoxifen treated cells almost eradicate alive leukemia cells on the ninth day in vitro (Figure C) and delay leukemogenesis in vivo (Figure D).
These findings suggest that ARHGEF12 as a transcriptional target of the AML1-ETO fusion protein and plays an essential role in leukemogenesis. The ARHGEF12-RHOA-ROCK pathway may serve as a new therapeutic target for AML1-ETO+ AML
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal